
|






|

|

Removal of Microorganisms (Sterilization)
Municipal and private water suppliers are extremely mindful of biological contaminants in their water sources. Municipal water companies absolutely and unequivocally need to kill all resident biological agents (e.g., bacteria, viruses, etc.) and hopefully thereafter to remove the residue thereof. Additionally, it is highly desirable to be able to place the sterilization technology far, far downstream in the local water distribution systems. Many developing countries’ water systems are so substandard and disintegrated that they simply cannot be expected to be impervious to biological contamination.
The most common sterilization method today is to "pour the chlorine" to the water to kill the microorganisms. When this occurs, people drink the dead bodies, the flotsam and jetsam of chlorination (a rather unappetizing prospect!), but those dead bodies cause no harm. They are biologically benign when they are dead. Reticle strives to improve over this approach. Technologies that kill microorganisms but do not remove their residue will be less valuable than technologies that remove all traces of them and their residue. Reticle does the trick.
There are two effective methods of sterilization that are supported by Reticle Carbon, methods that remove the microorganisms and all traces of their residues completely from the water stream.. The first method is to mineralize the microorganisms, to "zap" them into oblivion, right alongside the dissolved organics present in the water. Mineralization turns them into carbonic acid, obviously killing them in the process. This method always works, for it kills the organisms as they are chemically disintegrated.
The second method of sterilization offers even more promise. Microorganisms are encased in membranes (their "little skins"), which are neither simple nor inert. Quite the contrary, their "little skins" are complex and sophisticated organic membranes designed through eons of evolution to keep those organisms completely wet. How do they accomplish this? Simple--their membranes contain organic-inorganic chemical groups (amines, sulfonates, or similar chemical groups) that cause water ions (H+ and OH- depending on the pH of the water) to electrostatically stick to their skins and thereby to wet it (a process known as hydrophilicity), thereby electrostatically keeping the microorganism continuously wet.
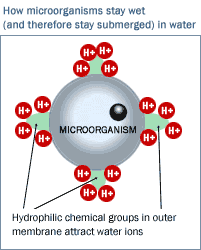 The figure illustrates that the wetting of small microorganisms is actually an electrostatic process. Keep in mind that if microorganisms were coated with non-polar organic molecules, they would float around like little oil slicks on the surface of the water, never being able to become wet. (They would be like the oil in your salad dressing, never becoming embedded or entrained in the water but merely floating on the surface.) They would dehydrate and die immediately. It is the hydrophilic polar components on their skins that attract water ions that allow them to immerse within the water mass. It is clear that once electrostatic wetting has occurred, the particle, i.e., the microorganism, is not at all an electrically neutral particle. The microorganism is in fact a charged particle, directly amenable to removal by Reticle Carbon CDI! It has a cluster of H+ ions or a cluster of OH- ions all around its "little skin." (This charge clustered around its skin, which is caused by inherent surface active chemical groups embedded in its skin, is called zeta potential, a phenomenon that is well known and well documented in the literature. Zeta potential pertains to many particles including but not limited to microorganisms.) The figure illustrates that the wetting of small microorganisms is actually an electrostatic process. Keep in mind that if microorganisms were coated with non-polar organic molecules, they would float around like little oil slicks on the surface of the water, never being able to become wet. (They would be like the oil in your salad dressing, never becoming embedded or entrained in the water but merely floating on the surface.) They would dehydrate and die immediately. It is the hydrophilic polar components on their skins that attract water ions that allow them to immerse within the water mass. It is clear that once electrostatic wetting has occurred, the particle, i.e., the microorganism, is not at all an electrically neutral particle. The microorganism is in fact a charged particle, directly amenable to removal by Reticle Carbon CDI! It has a cluster of H+ ions or a cluster of OH- ions all around its "little skin." (This charge clustered around its skin, which is caused by inherent surface active chemical groups embedded in its skin, is called zeta potential, a phenomenon that is well known and well documented in the literature. Zeta potential pertains to many particles including but not limited to microorganisms.)
As an intrinsically charged particle, the living microorganism is itself attracted directly to the charged Reticle Carbon electrodes via normal electrostatic attraction. To wit, microorganisms are charged particles (with an inherent, unique zeta potential) and are attracted to the electrodes and thereby removed directly from the water stream by Reticle Carbon CDI. Reticle Carbon CDI removes living microorganisms "thrashing and kicking" from a water stream directly, adsorbing them to the charged electrodes because of the zeta potential in their skins. This is a huge benefit—Reticle Carbon CDI directly sterilizes inbound water by directly removing entrained microorganisms in the water, concentrating them, and allowing them to be dispatched in concentrated form thereafter.
|

|

